Background:The advent of chimeric antigen receptor (CAR) T-cell therapy has changed the treatment landscape for refractory/relapsed B-cell lymphoma (r/r B-NHL). However, there are limited data on outcomes in older patients (age≥65 years) treated with CAR -T-cell therapy, particularly in patients older than 70 years.
Objectives:Our objective was to evaluate the efficacy of CAR -T-cell therapy in elderly patients with r/r B-NHL, including response rates, progression-free survival (PFS), and overall survival (OS), as well as factors affecting survival.
Methods:As of April 1, 2023, a total of 80 patients with r/r B-NHL in the age group 65-70 years (n=57) or older than 70 years (n=23) were included. Diagnoses included DLBCL NOS (n=58) ,HGBCL(n=6) ,TFL(n=6), Richter(n=4) and Other(n=6). Baseline characteristics are described in Table 1.All patients were heavily pretreated. 71/80 (88.75%) patients were at stage III-IV. The median IPI score was 3 (range 1-5). 8/96 (10%) patients failure of prior autologous hematopoietic stem cell transplantation (HSCT). The median assessment score of ADL scale was 95 for both the 65-70 and > 70 age groups, and 47.5% of patients received bridging therapy(BT) prior to CAR -T cell therapy. There were no significant differences in baseline data between age groups.
Prior to the study, CD19/CD22 antigen expression in tumor tissue was confirmed by pathology, and the target was selected according to antigen expression.
The kinetics and function of CAR -T cells were monitored by quantitative PCR and flow cytometry. Efficacy was assessed by PET-CT every 3 months after CAR -T therapy. All p-values were two-sided values. Survival curves were calculated by the Kaplan-Meier method.
Results: The number of patients who chose a CD19 target or a CD19/CD22 dual target for CAR -T therapy was 67/80 (%) and 13/80 (%), respectively. The median CAR -T cells infused dose in the 65-70 and > 70 age groups was 1.3 (range, 0.0485-9.5) and 1.6 (range, 0.088-3.91) (×106/Kg), respectively (P=0.9618).(Table 2).There were no differences between the 2 age groups in the occurrence of cytokine release syndrome (CRS) grade 3 or higher(P=0.6221). The proportion of patients using glucocorticoid therapy for CRS was higher in the age group > 70 years than in the age group 65-70 years, but there was no statistically significant difference (P=0.0957).
A response occurred in 35/57 (61.4%) of patients in the 65-70 years age group and in 14/23 (60.7%) of patients in the > 70 years age group (P=0.9646), with a complete response in 21/57 (36.8%) and 8/23 (34.8%), respectively (P=0.8623).
At a median follow-up of 8.05 months (95% CI: 10.27-15.20), 12-month overall survival(OS) was 54.6% (95%CI: 39.3%-67.6%) vs 72.1% (95%CI:43.9%-87.8%) at ages 65-70 vs > 70,12-month progression-free survival (PFS) was 32.8% (95%CI: 19.8%-46.5%) vs 32.9% (95%CI: 13.5%-54%), and there was no significant difference in OS (p=0.0623) and PFS(p=0.6327) by age group.
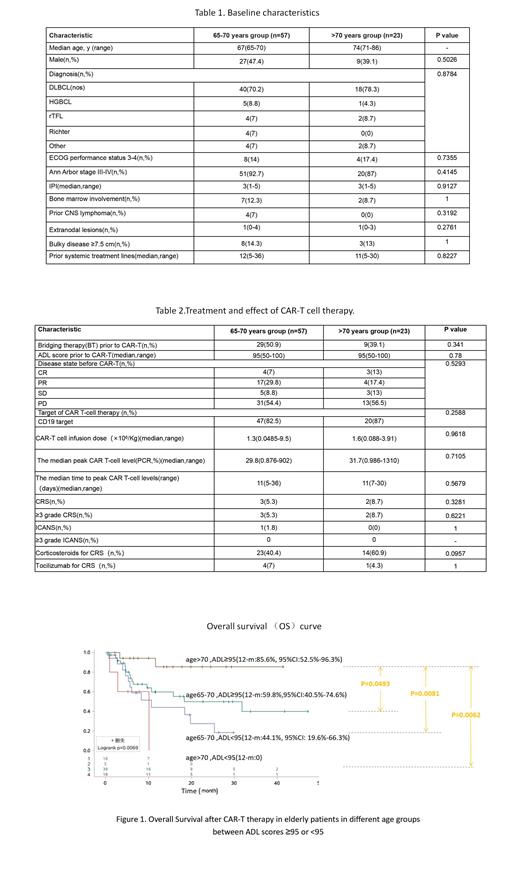
In contrast, patients aged > 70 with ADL < 95 showed significantly shorter 12-m OS (0) compared to patients aged 65-70 with ADL≥95(55.2%,95%CI:35.5%-71.1%) or ADL < 95(40.5%, 95%CI: 14.2%-59.9%), or > 70 with ADL≥95(85.6%, 95%CI:52.5%-96.3%), respectively (Figure 1: P= 0.0069).
The three negative factors comprising the prognostic tool OS: low ADL scores (P=0.0124), high IPI scores (P=0.0029), and presence of extranodal lesions (P=0.0143).
Conclusions: Our data suggest that the safety and efficacy of CAR -T-cell therapy in > 70-year-old patients is not significantly different from that in 65-70-year-olds. Elderly patients with ADL scores below 95 have poor survival after CAR -T therapy, especially those over 70 years of age. However, studies on curative approaches and long-term follow-up are needed.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal